What is the niPOC test on pregnancy remains?
- niPOC is an advanced non-invasive test which uses a blood test to determine whether a pregnancy loss was caused by a chromosome abnormality.
- Innovative application of non-invasive analysis of circulating foetal DNA for miscarriages.
Why is it so important?
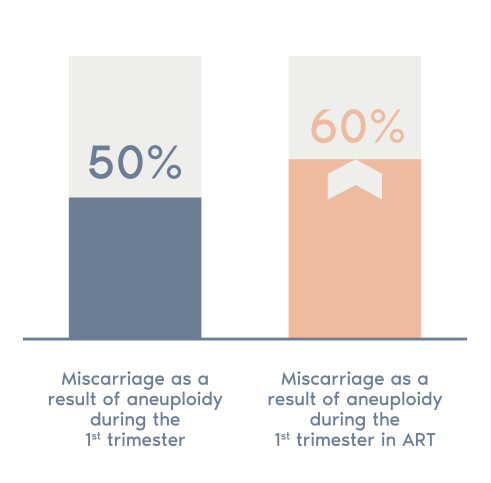
- 50% of miscarriages in the first term are due to chromosomal abnormalities.
- This figure rises to 60% in the case of women who receive assisted reproductive treatment and further increases with age.
- 24 chromosomes are screened to identify the reason for the miscarriage.
- The information provided by the niPOC test can help patients make future decisions on their reproductive health and maximize the probability of a successful pregnancy and reduce the likelihood of another miscarriage.
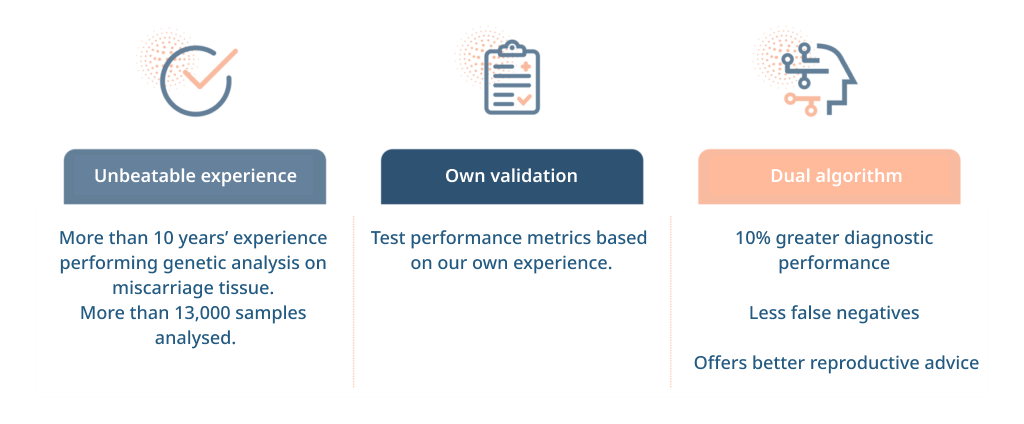
What is the procedure of the non-invasive POC test?
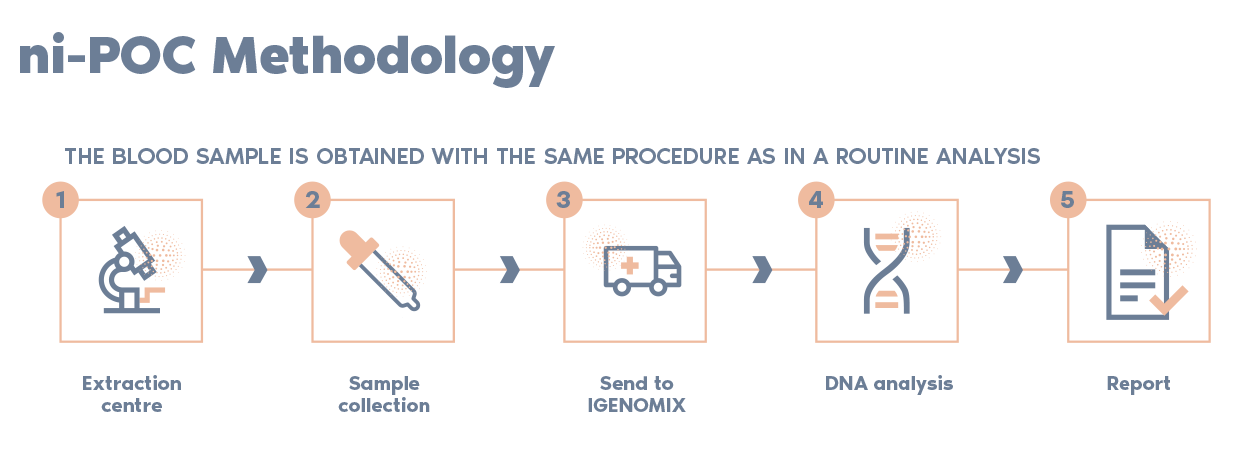
*This step must be performed first, before spontaneous expulsion, suction evacuation, curettage, or administration
of drugs to treat the expulsion or retained product of conception.