- Increases pregnancy rates per transfer:
Selecting normal embryos can increase the pregnancy chances after transfer
- Reduction in miscarriage rate:
In the general population, 25% of all clinical pregnancies end in miscarriage, the vast majority of which are due to aneuploidy
- Increase in the likelihood of having a healthy baby:
Some pregnancies with chromosomal anomalies can give rise to the birth of baby with a serious illness
- Reduction in time and necessary resources:
The time and resources necessary to achieve a pregnancy are reduced
- Reduces risk of multiple pregnancy:
A SET significantly reduces the likelihood of a twin pregnancy
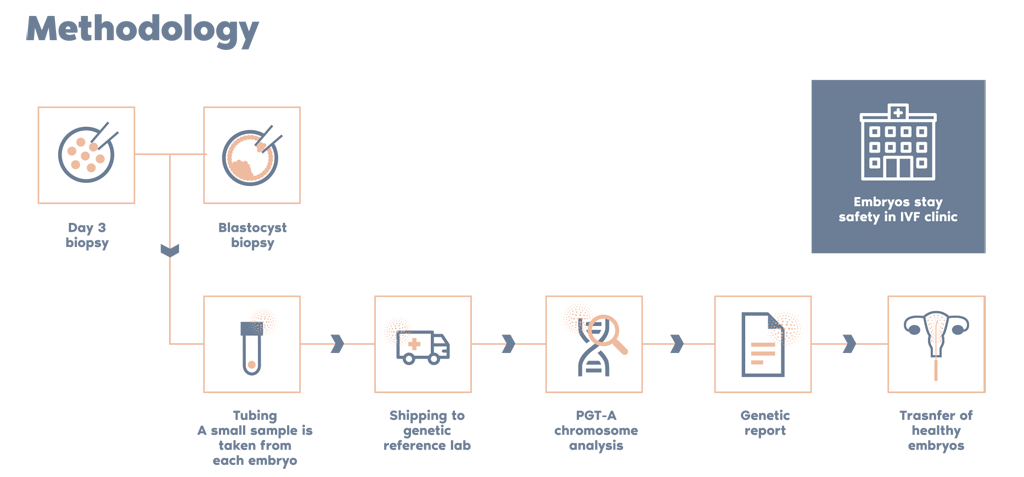
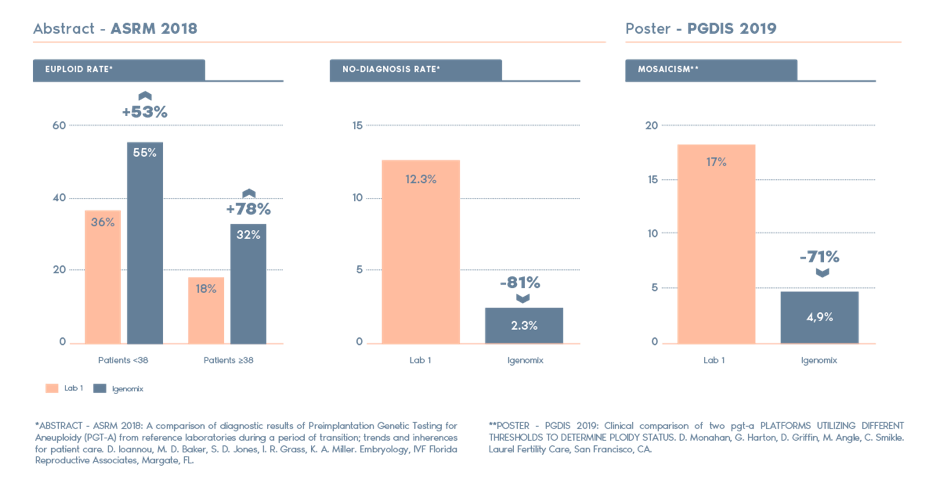
Why Igenomix PGT-A?
Independent studies have compared PGT-A from Igenomix and other labs and the highlights include:
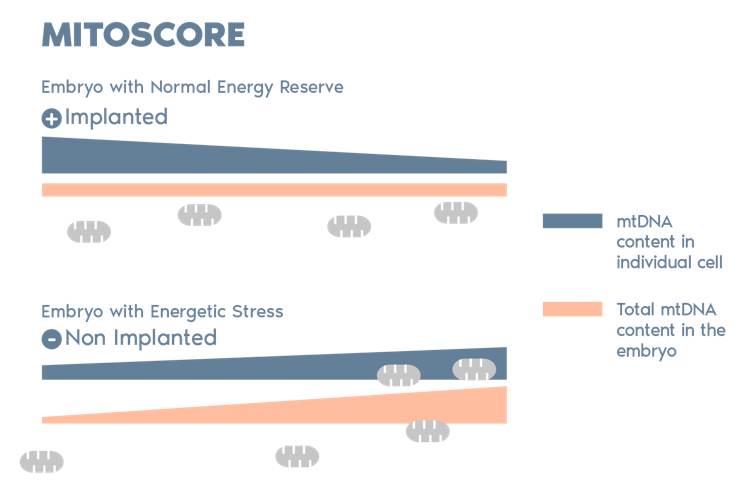
Igenomix Mitoscore
MitoScore is a mitochondrial biomarker developed by Igenomix, which gives us an indicator of the energy status of an embryo.
MitoScore allows us to select those embryos with the greatest probabilities for implantation that are more likely to result in a viable pregnancy through IVF/ PGT-A.*
*(Diez-Juan et al. 2015)
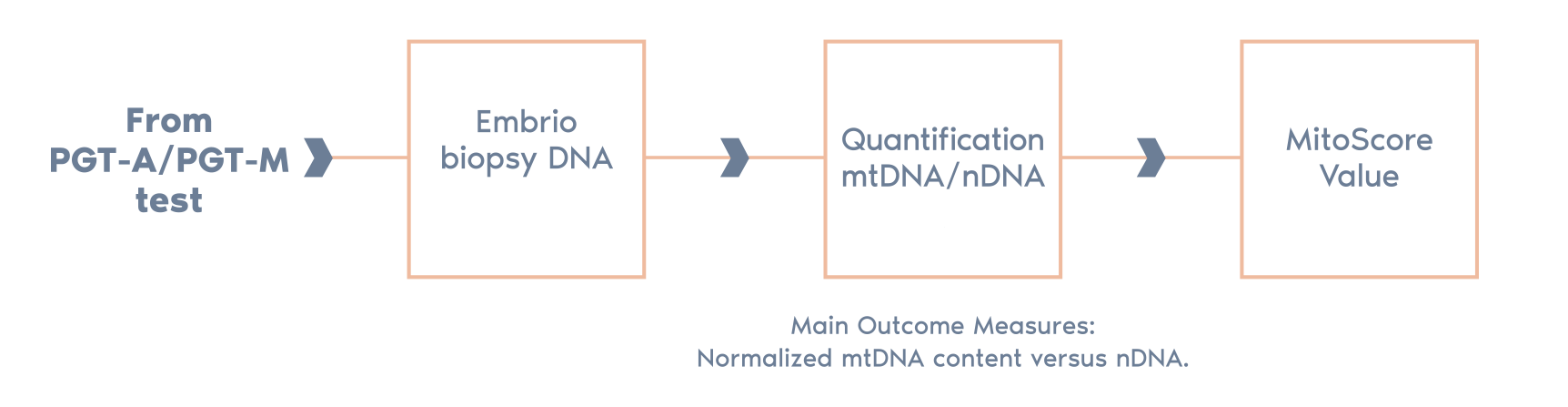
The clinical translation of this work is the integration of the mtDNA copy number (MitoScore) with the routine genetic analysis performed in the PGT-A process.
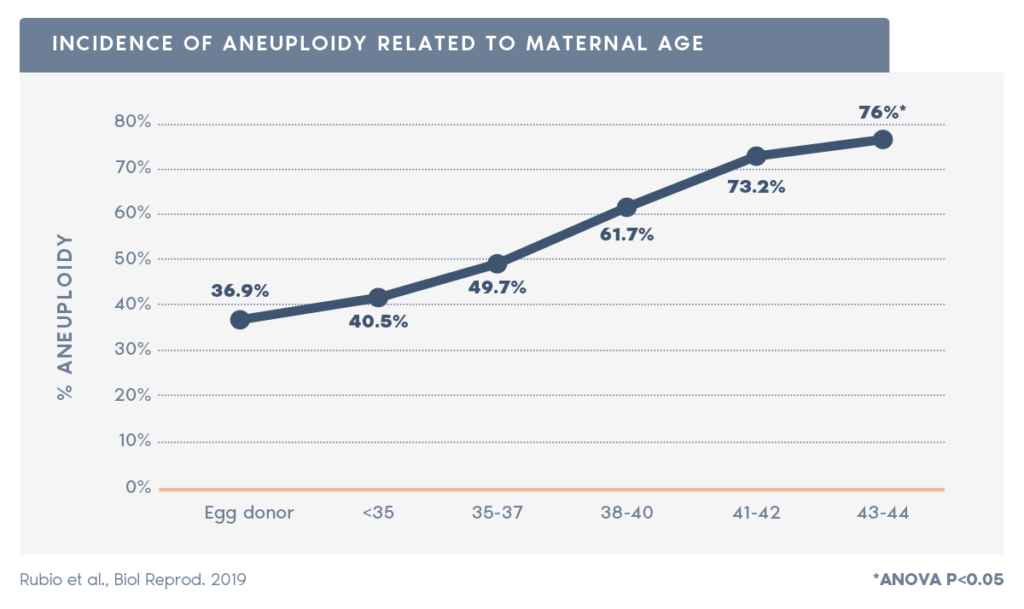
- PGT-A is particularly important for patients over 35, as aneuploidy rate increases with maternal age from approximately 52% at maternal age under 35, to approximately 80%, at age 42 (source:xxx).
- PGT-A also can greatly reduce the likelihood of a patient having a multiple-gestation pregnancy by choosing a Single Embryo Transfer (SET).
Test limitations
PGT-A does not test for:
Birth defects
Inherited single gene disorders, such as cystic fibrosis or Tay-Sachs disease
Multifactorial conditions, including autism
Adult-onset conditions such as diabetes or Alzheimer´s disease
Physical and mental traits, such as intelligence or athleticism
Microdeletions/microduplications
As with most tests, PGT-A has some limitations:
1. Accuracy is ~98%
- False positive: There is a small chance an embryo could be excluded unnecessarily
- False negative: There is a small chance that an embryo diagnosed as normal could still be chromosomally abnormal
2. PGT-A tests only the samples produced by embryo biopsy, not whole embryos
3. PGT-A does not detect structural abnormalities that do not involve gains or losses of genetic material. Additionally, the following cannot be detected:
- Chromosome losses/gains bellow 10Mb,
- Low level of mosaicism (<30%)
- Uniparental disomy (UDP)
- Defects affecting the complete set of chromosomes (haploidy, triploidy)
Follow-up prenatal testing is recommended to confirm the results of PGT-A.
There is a chance of unforeseeable problems with transportation, such as weather and air travel issues, or other circumstances beyond the control of Igenomix that may delay the reporting of results.
In a small percentage of cases, genetic testing cannot be performed due to improper biopsy techniques, loss of biopsied cells, or poor DNA quality.