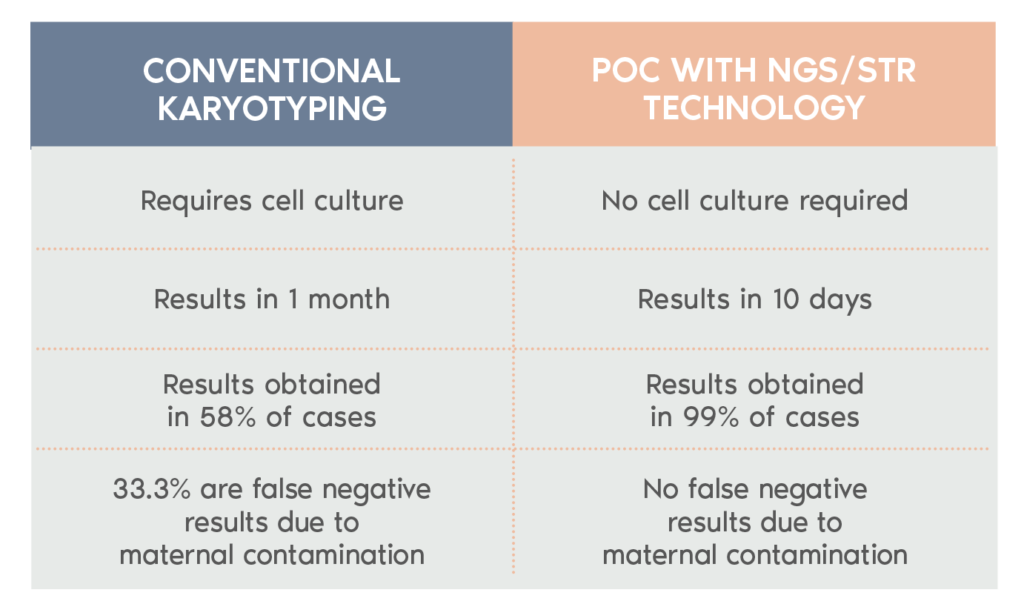
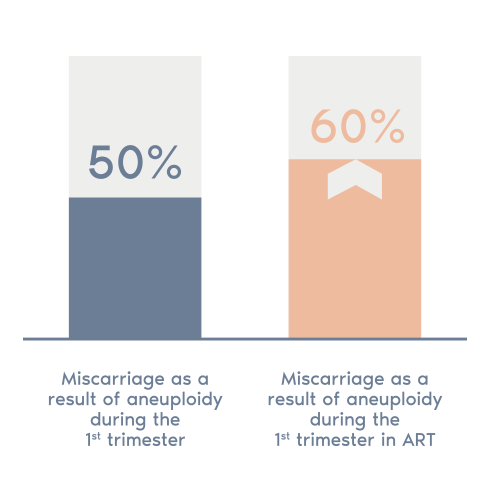
What is POC?
The POC test analyzes fetal tissues from a miscarriage to determine if the lost pregnancy was the result of a chromosomal aneuploidy.
This test can provide you an important information about the possible causes of your patient’s miscarriage to help them to plan any future pregnancy..
Methodology
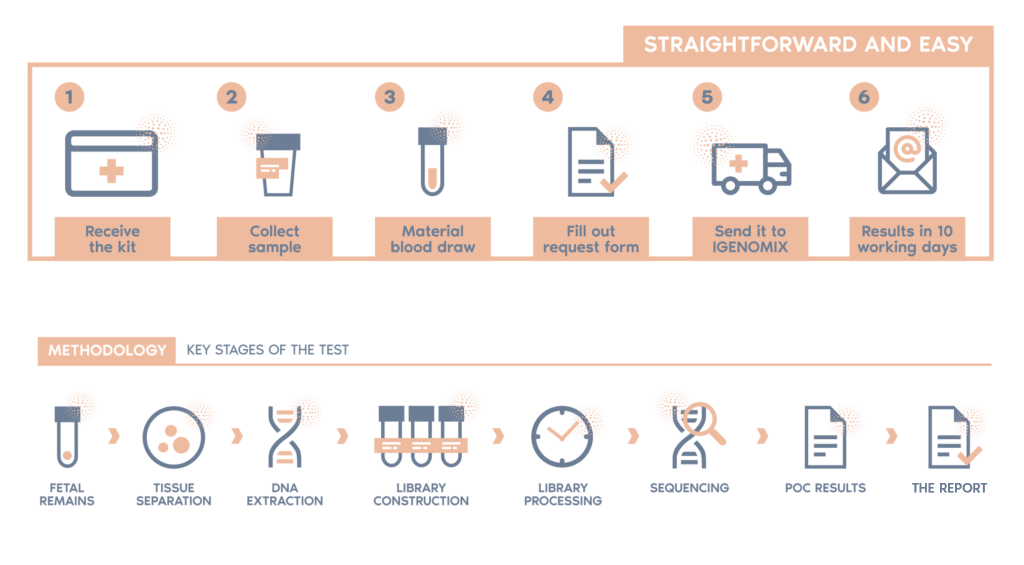
How to collect and send the sample
If a conventional curettage method is used, the sample should be collected in a sterile container and covered with saline, taking care to avoid contamination with maternal tissue as far as possible. The sample can also be obtained by directed biopsy following hystero-embryoscopy, and placed in a sterile 10 ml conical tube containing saline. 5 ml of maternal blood should be collected in an EDTA tube. Send the sample in the sealed container and maternal blood at room temperature and with suitable packaging to prevent damage. The sample should be sent as soon as possible to prevent tissue degradation. If there is a delay in sending the sample and blood, they should be refrigerated at 4ºC.