What is the EMMA – Endometrial Microbiome Metagenomic Analysis?
- EMMA is a test that analyses the endometrial microbiome to help identify abnormalities associated with a poor reproductive prognosis.
- EMMA assesses the endometrial microbiome, providing information about the most common and relevant bacteria, including those linked to higher pregnancy rates. EMMA also includes ALICE, which detects pathogenic bacteria that can cause chronic endometritis.
- Igenomix has analysed over 70,000 clinical samples during the last 5 years.
- Through our experience with the EMMA&ALICE tests, we have identified the bacterial pathogens most frequently detected in the endometrium.
- Based on this data, we have developed the most comprehensive and precise panel, encompassing all clinically relevant bacteria, with most identified at the species level, enabling a more precise and targeted treatments.
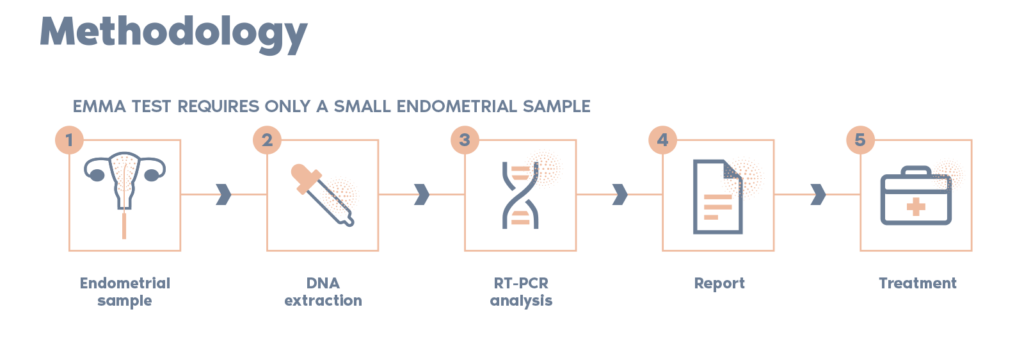
What is the procedure?