Neurodegenerative

Sample Requirements
For genetic testing through next generation sequencing, the following sample types are accepted. A thorough labelling of the tube with unique identifying information is suggested, incorrect labelling can lead to rejection of the sample. The minimum required information to identify and accept a sample is – Patient’s full name, Date of birth, Gender and Medical Record Number.
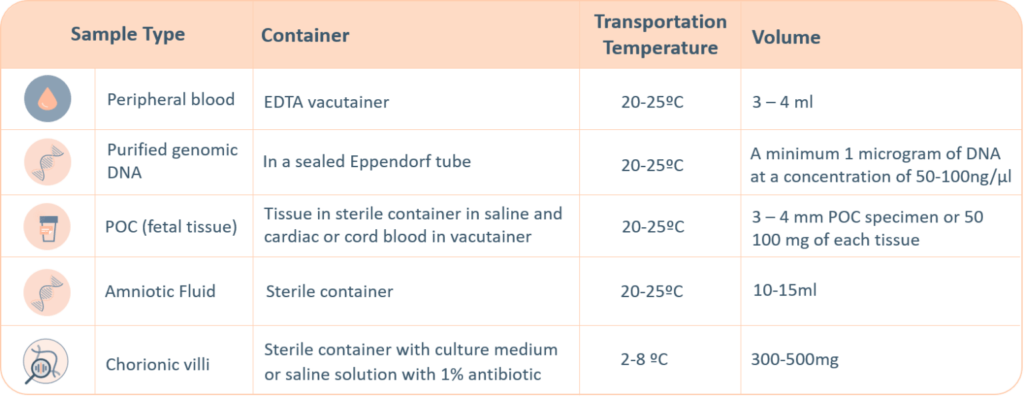
- Maternal blood sample must be sent with all products of conception, CVS and Amnio samples.
- Precedence will be given to all prenatal samples.
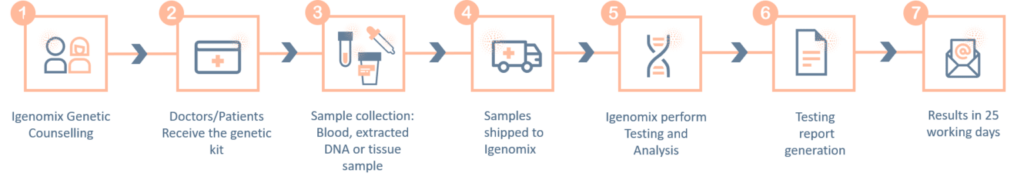
The ‘informed consent’ form and the ‘test requisition from’ (included within the provided kit) must be properly filled-in and signed by the patient and sent with the samples inside the shipping box or by e-mail to the laboratory. Igenomix will send you all the documents needed for the pick-up and transportation of the appropriate kit to our laboratory
Methodology
Limitations
The probes used for this test are designed to detect known genes in the curated panel. Therefore, this test is unable to detect genes not defined by the NCBI reference genome GRCh37 or non-human genome sequences including viral sequences or non-nuclear DNA that are designated in the specific panel.
In addition, due to the limitations of NGS technologies, the following variants cannot be readily detected: large deletions/duplications greater than 40 base pairs, copy number variations, homopolymer stretches, variants in pseudogene regions, gene fusions, balanced translocations, inversions, ploidy changes, uniparental disomy, and repeat expansion regions.
Furthermore, variants present outside the exons (non-coding region) could be missed; these variants can affect gene activity and protein production which may lead to genetic disorders. This technique does not cover the entire exome, (the % of bases with coverage above 20x is approximately 97%). It may not be possible to resolve certain details about variants such as mosaicism, phasing, or mapping ambiguity.
Analytical limitations may also occur due to the provided clinician information. Accurate and thorough clinical information of the patient(s) and family members is required as incomplete information may lead to false positive or negative results





