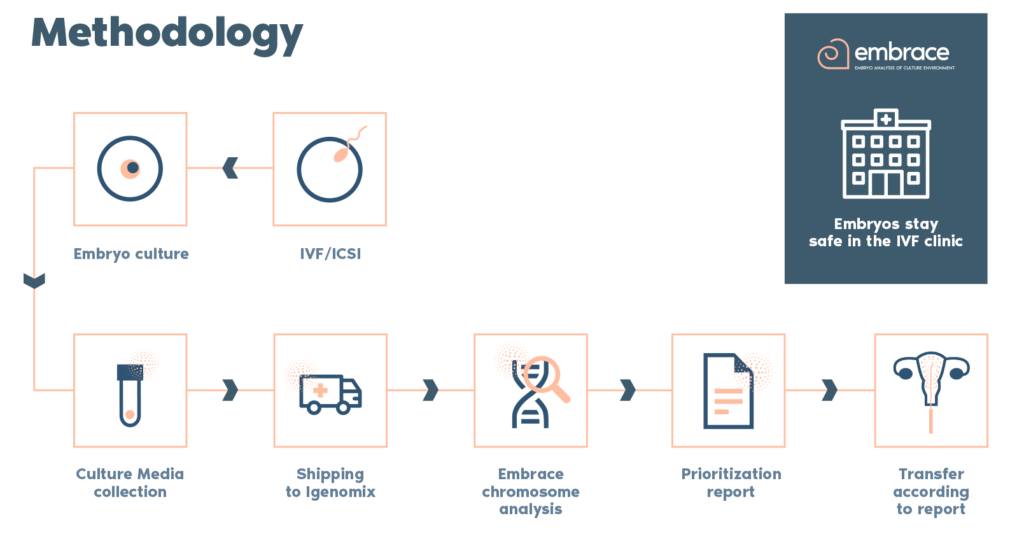
Why use EMBRACE test?
- It avoids embryo biopsies and therefore reduces costs, making it accessible to a greater number of people.
- A non-invasive solution for patients, leading to safer, more efficient IVF treatment.
Scientific Evidence
This new test is based on our recent study ‘Multicenter prospective study of concordance between embryo cell-free DNA and trophectoderm biopsies from 1,301 human blastocysts’ published in AJOG.
- This is the largest study to date assessing ploidy concordance per embryo between invasive and non-invasive PGT-A.
- The study had two main aims – to evaluate the concordance and reproducibility of testing embryo cell-free DNA versus trophectoderm DNA obtained from the same embryo in a large sample of day 6 and day 7 blastocysts, and to assess the concordance rates with the inner cell mass of the blastocyst in a subset of blastocysts donated for research.
- It is the only truly non-invasive protocol, without any previous manipulation of the embryo. No previous assisted hatching, biopsy or vitrification was permitted before media collection.
- Embryo cell-free DNA analysis shows high reliability in a multicentre study involving different culture and clinical conditions including: different patient clinical backgrounds, ovarian stimulation protocols, culture media, incubators and embryo quality.
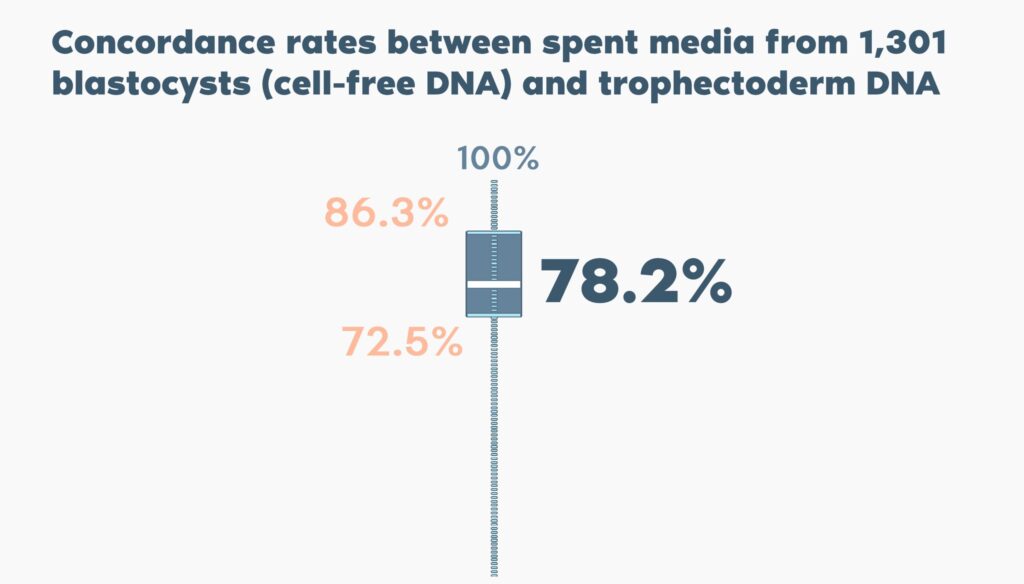
The concordance rate was on average 78.2% (ranging from 72.5% to 86.3% in different centres), with no significant between-centre differences related to culture conditions or blastocyst quality.
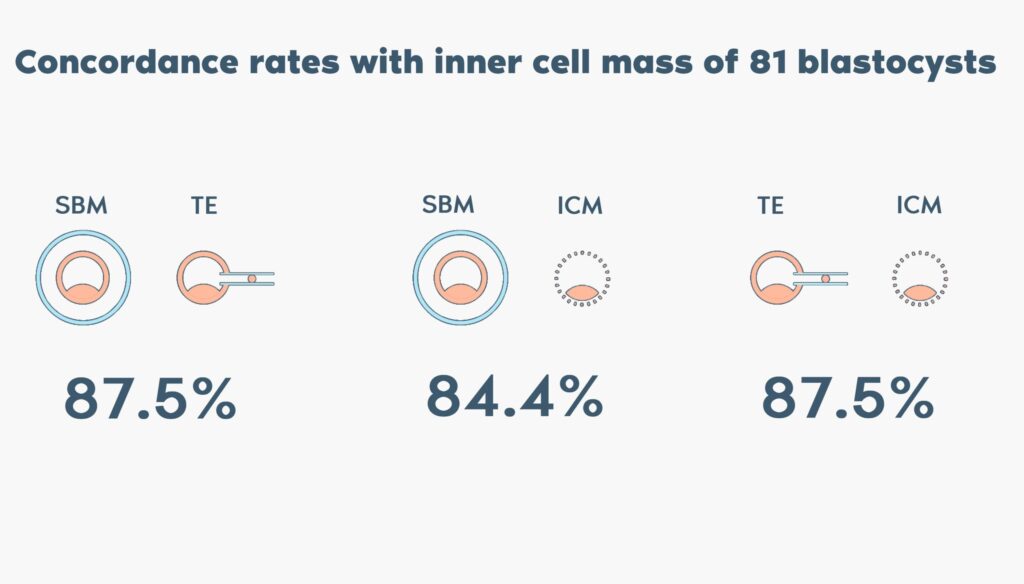
In addition, in a subgroup of 81 blastocysts, comparison of the inner cell mass (ICM) with the embryo cell-free DNA in spent blastocyst medium (SBM) and the trophectoderm (TE) biopsies has shown similar concordance rates, 84.4% and 87.5% respectively.